In the intricate and meticulous process of drug development, the presence of impurities, such as Gemigliptin Impurity 3, plays a multifaceted role that is as critical as it is complex. This article aims to explore the significance of Gemigliptin Impurity 3, its sale, and the role it serves in the broader context of pharmaceutical research and manufacturing.
Understanding Gemigliptin Impurity 3
Gemigliptin is a DPP-4 inhibitor used in the treatment of type 2 diabetes, and its purity is paramount to its efficacy and safety. Gemigliptin Impurity 3, one of the potential impurities associated with the drug, is a byproduct of the synthesis process. It is characterized by a unique molecular formula and weight, which are crucial for understanding its chemical behavior and potential impact on the drug's performance.
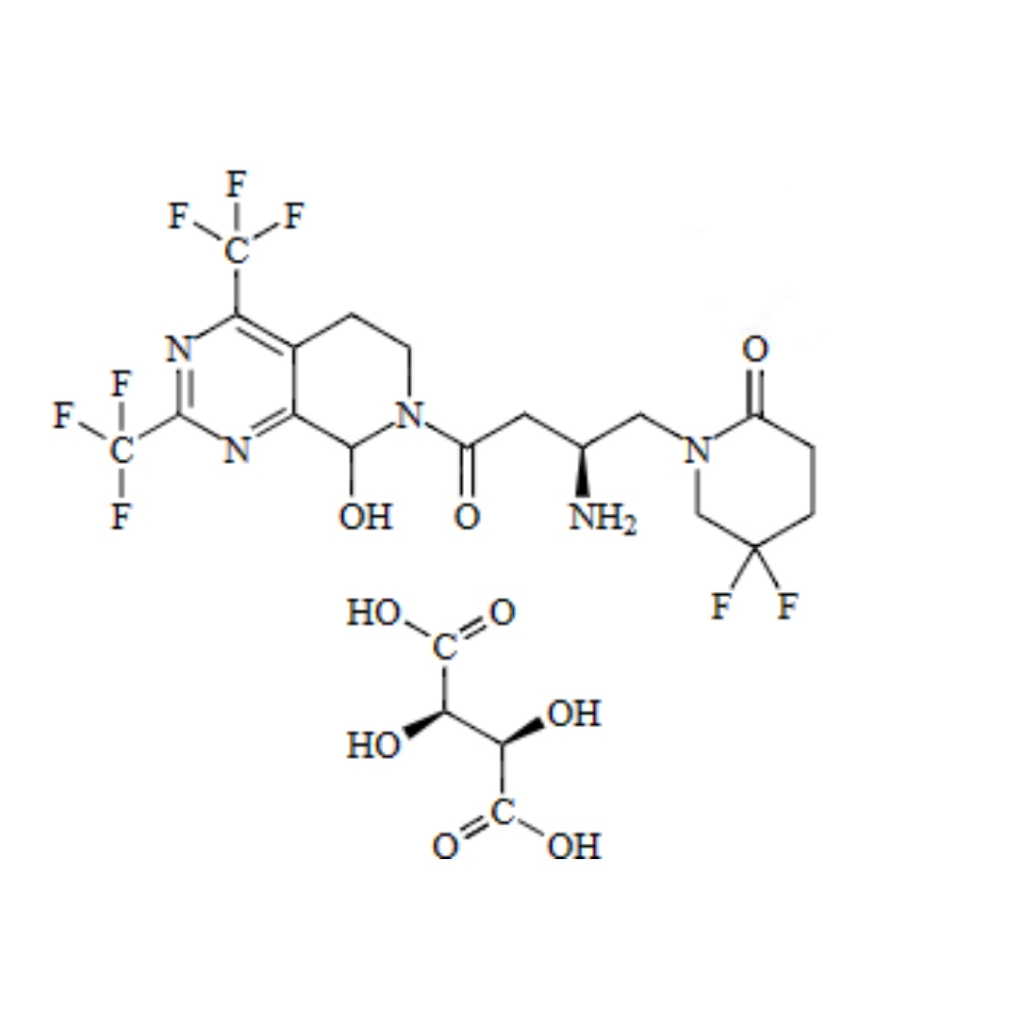
The Role of Gemigliptin Impurity 3
The role of Gemigliptin Impurity 3 in drug development is twofold: as a quality control parameter and as a potential source of information for improving the synthesis process.
-
Quality Control: The presence of Gemigliptin Impurity 3 must be monitored and controlled to ensure that the final drug product meets the stringent purity requirements set by regulatory agencies. This is essential for patient safety and drug efficacy.
-
Process Optimization: By studying the formation and characteristics of Gemigliptin Impurity 3, researchers can gain insights into the synthesis process. This knowledge can lead to the development of more efficient and cleaner methods to produce Gemigliptin, reducing the formation of impurities and improving the overall yield of the desired compound.
Sale of Gemigliptin Impurity 3
The sale of Gemigliptin Impurity 3 is primarily targeted towards research institutions, pharmaceutical companies, and regulatory bodies involved in drug development. It serves as a reference material for method development, validation of analytical techniques, and as a control in the quality assurance process.
Gemigliptin Impurity 3 Provider
A reliable Gemigliptin Impurity 3 provider is essential in the pharmaceutical industry. Such providers must meet several criteria:
-
Quality Assurance: The provider must ensure that the impurity meets the required purity standards and is accompanied by a certificate of analysis.
-
Regulatory Compliance: The provider should be aware of and adhere to the regulatory requirements for the sale and distribution of pharmaceutical impurities.
-
Customization: Some clients may require specific quantities or forms of the impurity. A good provider will be able to offer customization services to meet these needs.
-
Technical Support: Providing technical support and consultation can be a significant advantage for a Gemigliptin Impurity 3 provider, assisting clients in their research and development efforts.
The Importance of a Gemigliptin Impurity 3 Provider
A Gemigliptin Impurity 3 provider plays a pivotal role in the drug development process. They are not only suppliers of a specific chemical compound but also partners in ensuring the quality and safety of the final drug product. By offering high-quality impurities and supporting services, these providers contribute to the advancement of pharmaceutical research and the development of life-saving medications.
SACH: A Leading Gemigliptin Impurity 3 Provider
SACH is a renowned provider of Gemigliptin Impurity 3, committed to delivering the highest quality impurities for pharmaceutical research and development. With a focus on quality assurance, regulatory compliance, and customer satisfaction, SACH has established itself as a trusted partner in the industry.
SACH's commitment to excellence is evident in the services they provide, including:
-
High-Quality Impurities: SACH ensures that Gemigliptin Impurity 3 is supplied with the utmost purity, accompanied by a comprehensive certificate of analysis.
-
Customization Services: Understanding the unique needs of their clients, SACH offers customized solutions to meet specific research and development requirements.
-
Technical Expertise: With a team of experts, SACH provides valuable technical support and consultation to assist clients in their analytical and synthesis endeavors.
Conclusion
In conclusion, the role of Gemigliptin Impurity 3 in drug development extends beyond being a mere byproduct of the synthesis process. It is a critical component in the quality control and process optimization of Gemigliptin. The sale of this impurity is a specialized market, catering to the needs of the pharmaceutical industry for research and regulatory compliance. Providers like SACH are essential in this ecosystem, ensuring that the impurities are available for research purposes and that they meet the high standards required for use in drug development.
The role of Gemigliptin Impurity 3, its sale, and the providers that supply it, such as SACH, are all interconnected and play a vital role in the complex web of pharmaceutical development. As the industry continues to evolve, so too will the methods and standards for dealing with impurities, ensuring that the drugs we rely on are as safe and effective as possible.
www.hzsqchem.com
SACH